Team of 10 water molecules holds insulin hexamer together
Insulin is the most potent drug for treatment of Diabetes. Naturally, study of the structural integrity of this drug molecule is of utmost importance. In this work, for the first time, we investigate the role of water molecules in stabilizing the hexameric form of insulin, which acts as the storage unit of the biologically active monomers. Both X-ray crystallography and computer simulations (molecular dynamics) indicate the presence of approximately 10 water molecules inside a barrel-shaped cavity at the centre of the insulin hexamer assembly. We find that these water molecules display slow dynamical features and weave an intricate hydrogen bond network (HBN) amongst themselves involving the neighbouring amino acid side-chains. This HBN acts as a robust backbone that holds the bio-assembly from within. Furthermore, the water molecules inside the cavity effectively screen the charges on the side-chains and Zn2+ ions that wall the cavity, thus preventing a fatal collapse due to attractive/repulsive interactions. Simulations show that in absence of water molecules, the cavity collapses within a few picoseconds, destroying the structure and symmetry of the whole hexamer. This ultimately affects the functioning of the biomolecule. Our work provides yet another instance of the importance of water in biology.
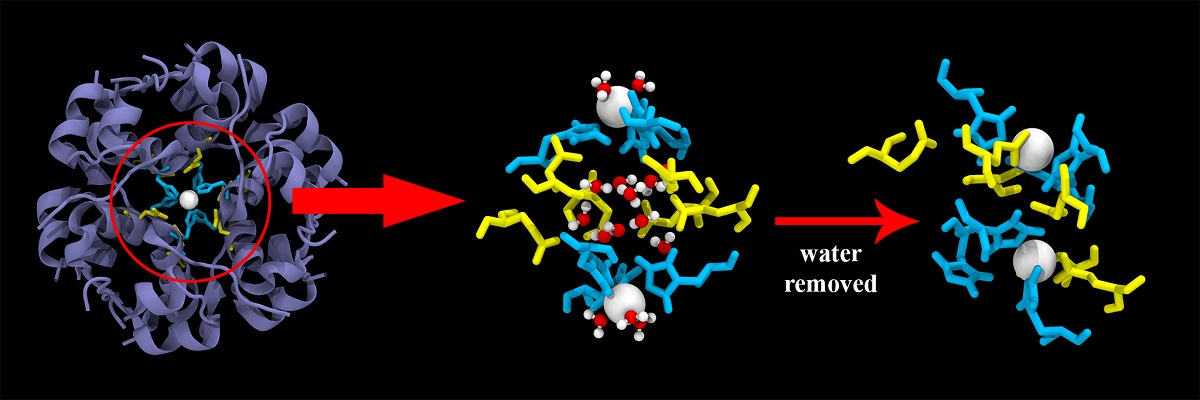
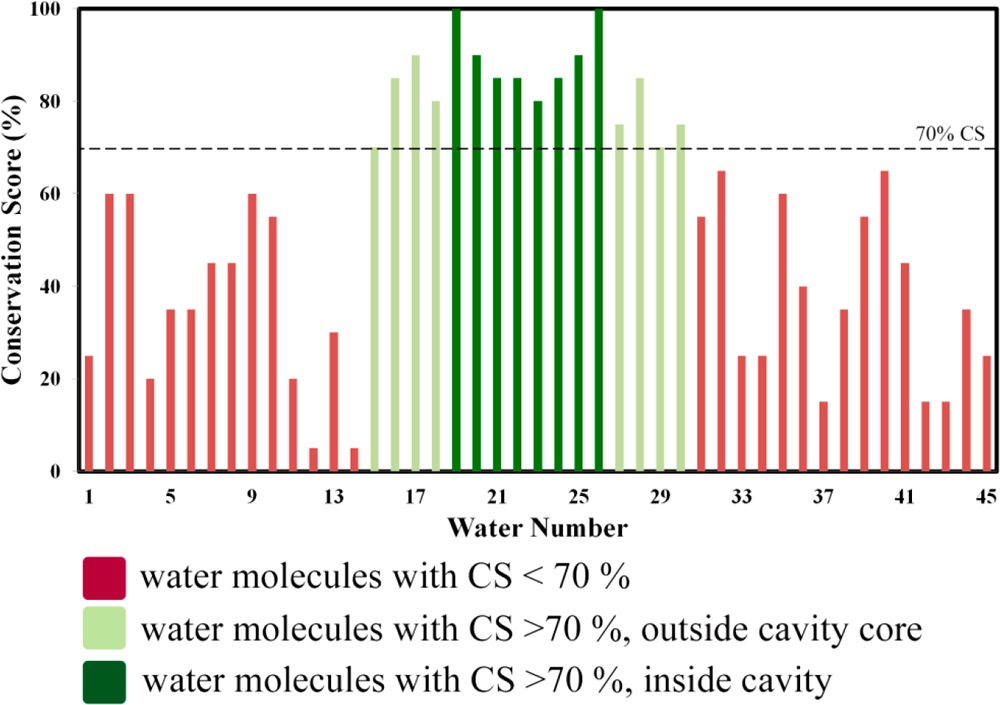
Figure 1. Conservation scores of water molecules in an insulin hexamer. Dark green lines represent water molecules inside the core of the cavity. Light green lines are for the molecules which are hydrogen boded to protein residues but do not reside at the core of the cavity. Red lines indicate water outside of the cavity.
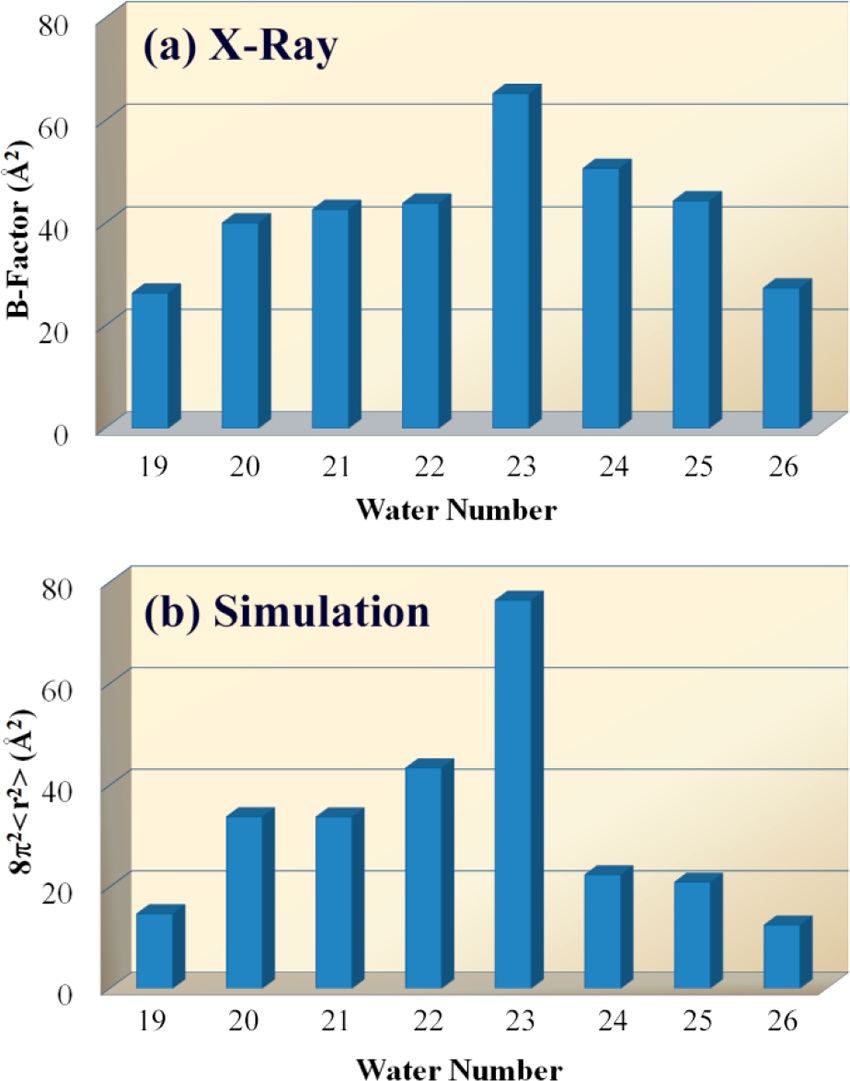
Figure 2. B-Factor analysis of cavity waters from (a) X-ray crystallography and (b) analogous analysis in computer simulation.
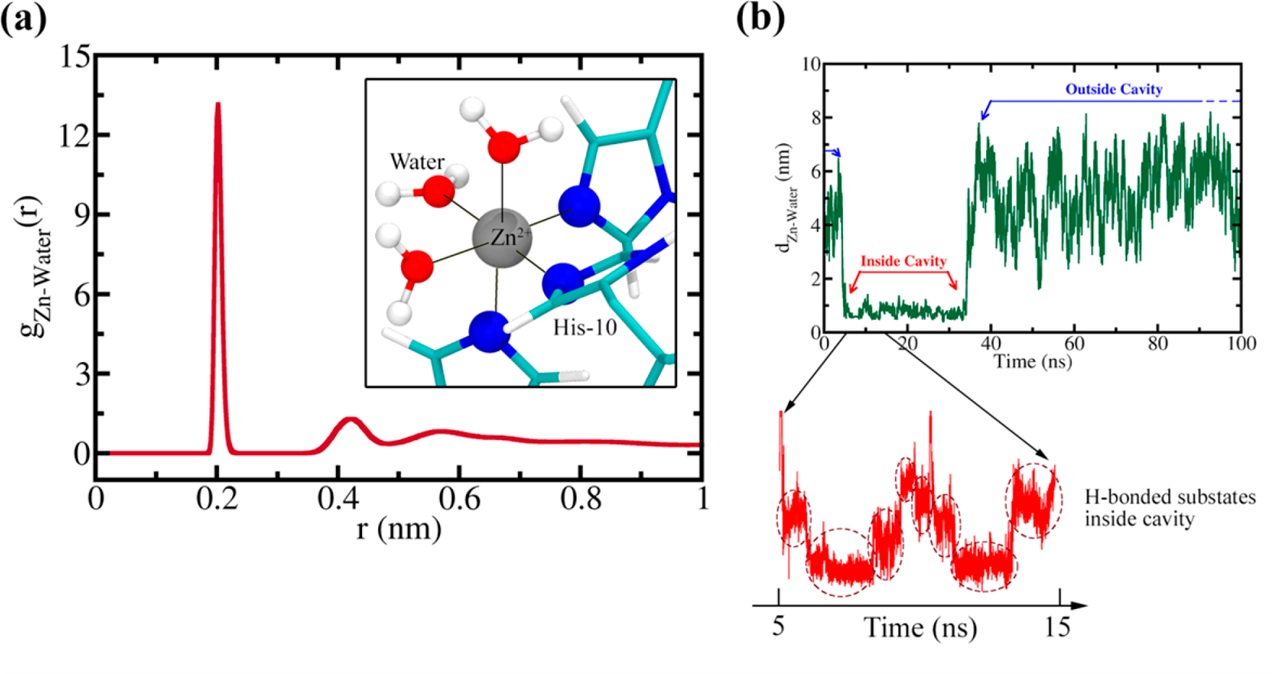
Figure 3. (a) Pair correlation function between Zn2+ ions and water oxygen [gZn−water(r)]. The Sharp peak at 0.20 nm corresponds to the three coordinated water molecules that create an octahedral atmosphere around each Zn2+ ion along with three His-10 residues (inset). (b) Distance of a tagged water molecule from a Zn2+ ion (dZn−water) for a 100 ns trajectory. From 5 to 35 ns, the water resides inside the cavity, denoted by a steady decreased distance from the Zn2+ ion. Inside the cavity, the water molecule attains certain stable H-bonded substates (circled by dashed lines) (represented for duration of 10 ns here).
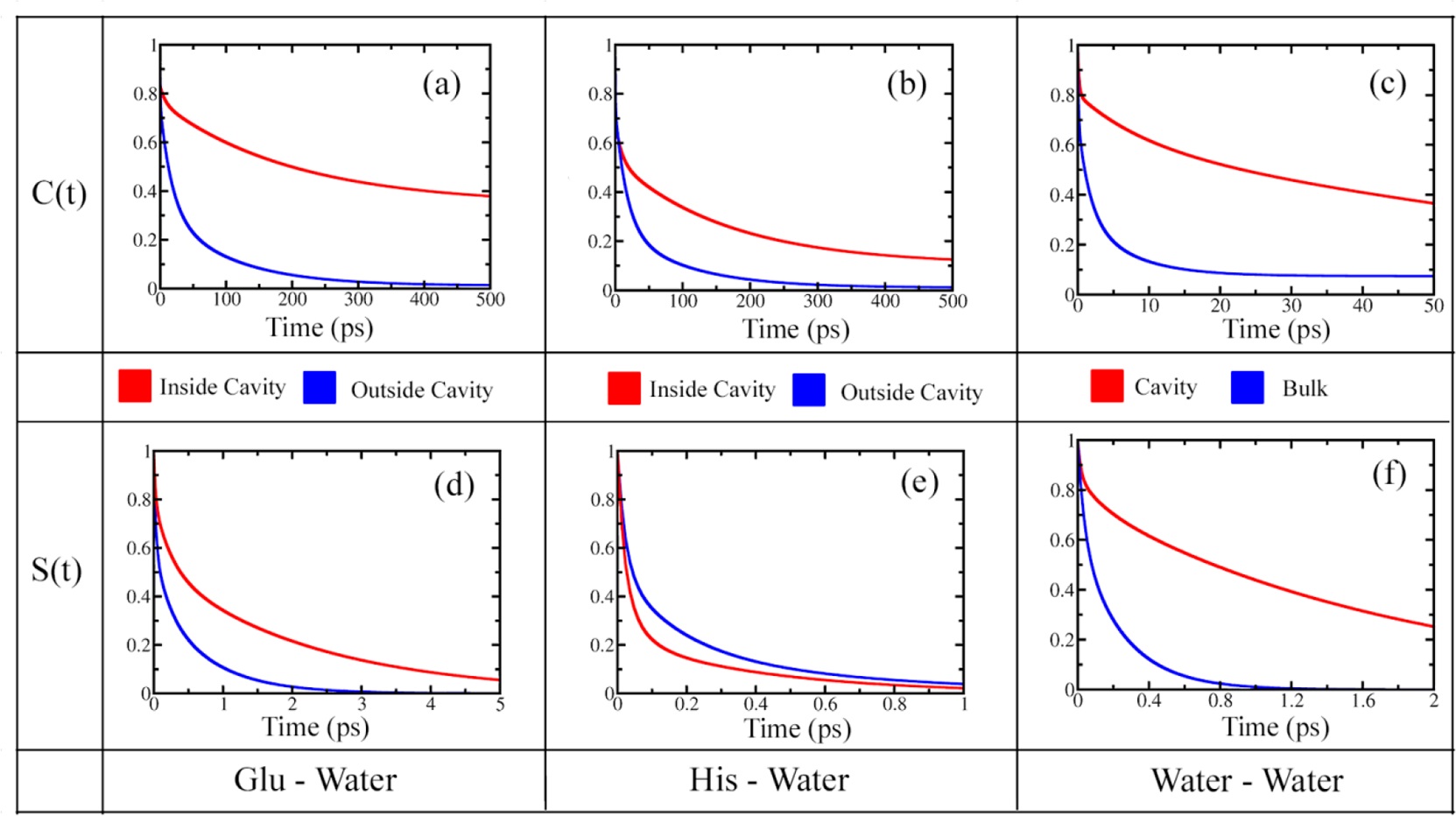
Figure 4. HB time correlation functions of water molecules among themselves and nearby protein residues. (a) C(t) for glutamate-water HB; (b) C(t) for histidine-water HB; (c) C(t) for water-water HB; (d) S(t) for glutamate-water HB; (e) S(t) for histidine-water HB; (f) S(t) for water-water HB. Red curves are for the dynamics inside the cavity, and blue curves represent the same outside or in the bulk.
References:
- Mukherjee, S.; Mondal, S.; Deshmukh, A. A.; Gopal, B.; Bagchi, B. “What gives insulin hexamer its unique shape and stability? Role of ten confined water molecules” Phys. Chem. B 2018, 122, 1631-1637.(pdf)(Poster)
- “Insulin falls apart without water” c&en News 2018, 96(5), 11.(pdf)








