Pannexins as conduits of cellular ATP release
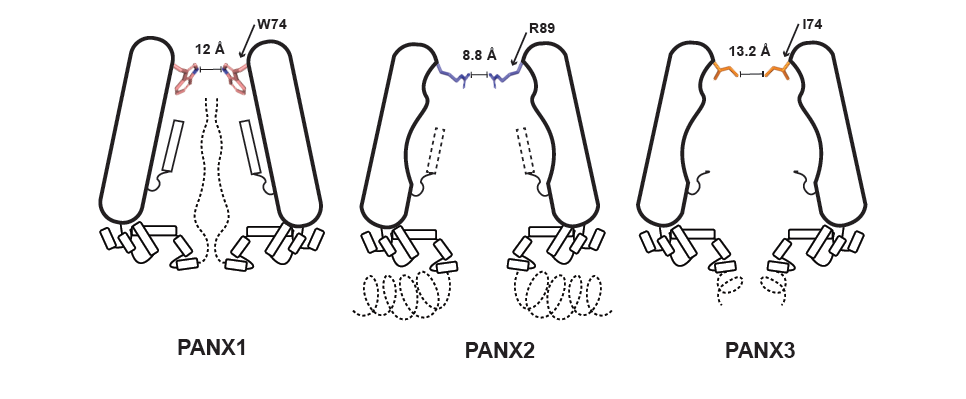
Figure legend: Basic organisation of the three pannexin isoforms (Credit: Nazia Hussain )
Ion channels in cell membranes allow small ions to move through them. Some channels, however, can allow larger molecules like ATP, the energy currency of the cell. ATP also plays key roles in signalling; for example, dying cells release ATP as a “find me” signal for macrophages to ingest them.
The release of ATP happens through a group of large-pore ion channels referred to as pannexins, which are not selective to other ions. Pannexins play an important role in ATP-mediated signaling, and have been linked to inflammatory conditions like gout and neuropathic pain – drugs used to treat gout, like carbenoxolone, seek to target these pannexins.
Pannexins are found in three isoforms in diverse cell types. Pannexin 1 is the most widely found and studied form, and is involved in ATP release in response to caspase activation. Pannexin 2 is largely a neuronal isoform that has a long disordered region. Pannexin 3 is the shortest isoform and is involved in bone and cartilage development. The three versions resemble an inverted cone with a narrow pore at the top facing the outside environment and a wider vestibule towards the cell’s interior. The narrow pore constricts ion and ATP movement, and is lined by different residues in each isoform.
In a new study, researchers from IISc led by Aravind Penmatsa used cryo-electron microscopy and electrophysiology techniques to delve into the inner workings of these isoforms. They found that the pore in pannexin 1 has a large aromatic residue, while pannexin 3 has a short hydrophobic residue. As a result, pannexin 3 has a wider pore and is less responsive to inhibition by carbenoxolone, a pannexin 1 inhibitor. Pannexin 3 also has another constriction in the vestibular chamber that further impacts the entry of molecules into the channel. Modifications to the pannexin 1 pore to resemble pannexin 2 causes further constriction as pannexin 2 has positively-charged arginine in its pore. The substitutions also weaken the electrical properties of the channel and its ability to interact with ATP.
The team also noticed that a germline mutant of pannexin 1 at a distant site from the pore causes the aromatic side chain to move, thereby constricting it. This closure can dramatically affect ATP release, with the channel losing its ability to conduct ions due to the mutation. Patients with this pannexin mutation have severe mental disabilities, hearing loss and reduced growth.
The findings underscore how changes in structure alter the pannexins’ ability to aid in ATP signalling.
REFERENCE:
Hussain N, Apotikar A, Pidathala S, Mukherjee S, Burada AP, Sikdar SK, Vinothkumar KR, Penmatsa A, Cryo-EM structures of pannexin 1 and 3 reveal differences among pannexin isoforms, Nature Communications (2024).
doi: 10.1038/s41467-024-47142-6
The study was funded by the MoE-STARS programme and the DBT-Wellcome Trust India Alliance.
WEBSITE:
https://aplabmbu.weebly.com/
Twitter handles: @PenmatsaLab; @nazia_hussain7; @MBU_IISc;




