Origin of n-type conductivity of monolayer MoS2
Monolayer (ML) molybdenum disulfide (MoS2) is a promising two-dimensional material for the energy efficient field-effect transistors and flexible nano-optoelectronic devices. As-grown MoS2 is an n-type semiconductor; however, the origin of this unintentional conductivity is still not clear. Using hybrid density functional theory, we have shown that the commonly observed native point defects and previously considered S vacancy do not lead to n-type conductivity in ML MoS2. Interestingly, hydrogen which is ubiquitous and is always present in almost all of the growth environments is found to be the most stable in its interstitial (Hi) and H-S adatom (hydrogen on the top of S atom) forms, in ML MoS2. The defect thermodynamic analysis shows that Hi and H-S adatom are shallow donor defects, with low formation energies and are the reason for n-type conductivity in ML MoS2. Furthermore, the calculated energy barrier height to move hydrogen from one position to another equivalent position is found to be more than 1 eV, which ensures their stability even at the higher growth temperature. Therefore, the presence of hydrogen leads to the n-type conductivity in ML MoS2.
Reference:
A. Singh and A. K. Singh, Origin of n-type conductivity of monolayer MoS2, Phys. Rev. B: Rapid Comm. 99, 121201(R) (2019)
Website: http://mrc.iisc.ac.in/abhishek/
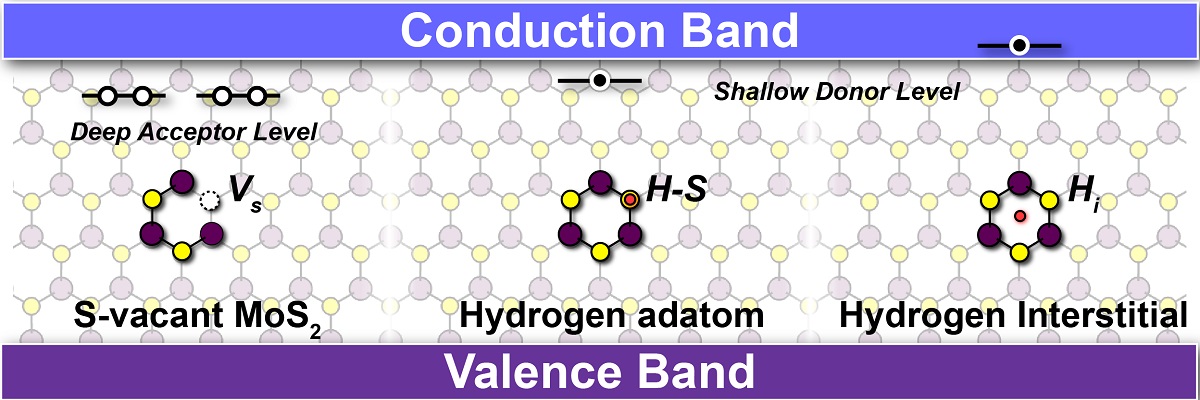
Origin of n-type conductivity of monolayer MoS2; Sulfur vacancy (VS) in ML MoS2, which has been widely attributed to an n-type conductivity, provide the deep acceptor levels near the conduction band and hence cannot be the cause of n-type conductivity. Hydrogen, which is almost always present in the growth environments, is most stable in Hi and H-S adatom, which provide shallow donor levels leading to the n-type conductivity in ML MoS2.
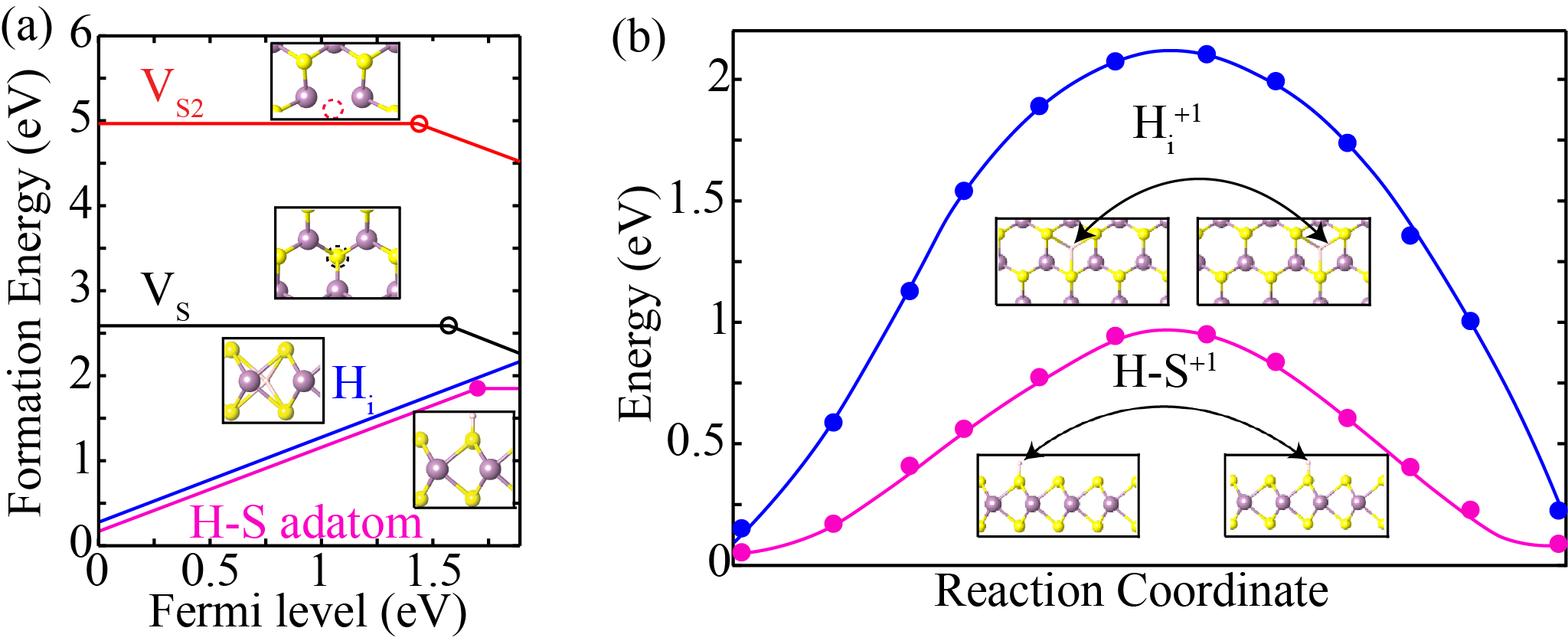
Defect formation energy as a function of Fermi-level (EF); for Sulfur mono and di-vacancy (VS and VS2), hydrogen interstitial (Hi), and H-S adatom in (a) S-rich growth condition (b) energy barrier height of Hi and H-S adatom. The energy barrier height of Hi (blue colour) and H-S adatom (magenta colour) are 2.1 eV and 0.9 eV, respectively, which ensure their stability even at the higher growth temperature.







