Building strong “highways” at the cellular scale
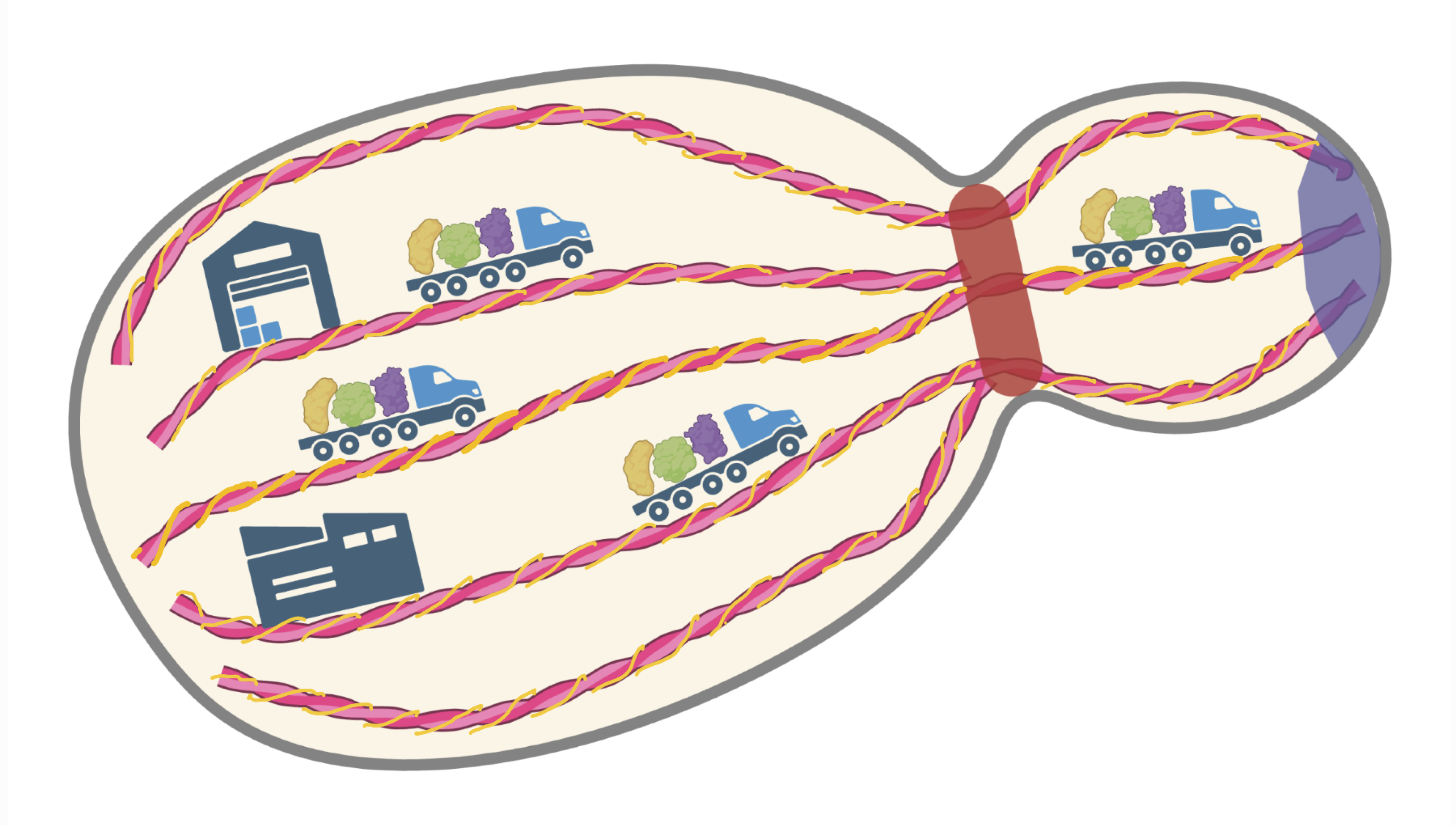
A simplified cartoon representation of a budding yeast cell showing actin filaments (red) decorated by Tropomyosin (yellow) that together act as “highways” for transport of cellular cargo towards the growing part of the cell (Created in BioRender. https://BioRender.com/ledl000. Credits: Anubhav Dhar, Saravanan Palani)
Imagine every cell as a city with a highway system built from a protein called actin. These highways are essential for transporting supplies around the cells, but are inherently unstable. To stabilise them, cells use a “guardrail” protein called tropomyosin. In budding yeast, a model organism for studying fundamental biology, two types of tropomyosin, Tpm1 and Tpm2, are present in different amounts. Over the years, research has shown that Tpm1, the more abundant tropomyosin type, stabilises the actin “highways” and is vital for normal cell growth and fitness. On the other hand, Tpm2, present at about 1/5th of the amount of Tpm1, was believed to not play a role in maintaining stability of the actin filaments.
A new study led by Saravanan Palani in the Department of Biochemistry, IISc, challenges this long-held belief. Using a strategy that allowed the team to track the location of these tropomyosin proteins in real time in living cells, they show that the two types of tropomyosin actually work together to stabilise the cell’s internal actin filament network. The researchers increased the amount of Tpm2 in the cells that lacked Tpm1 and discovered that increased amounts of Tpm2 could fully substitute for all known functions of Tpm1. These observations reveal a hidden shared capability among these tropomyosin types present in yeast. The study uncovers a potential back-up mechanism for cells to ensure their transport system remains robust and functional even under stress.
This discovery has profound implications beyond yeast. Humans also have different tropomyosin proteins that are crucial in our muscles and nearly every other cell type. When these proteins malfunction, they are linked to serious diseases like cancer, cardiomyopathy, and genetic muscle disorders. This study provides a powerful new direction, suggesting that our own tropomyosin proteins might also have overlapping functions. Understanding this shared capability could open new avenues for treating diseases caused by a single faulty protein. By revealing a fundamental principle of cellular resilience in yeast, this research paves the way for new questions about human health and disease.
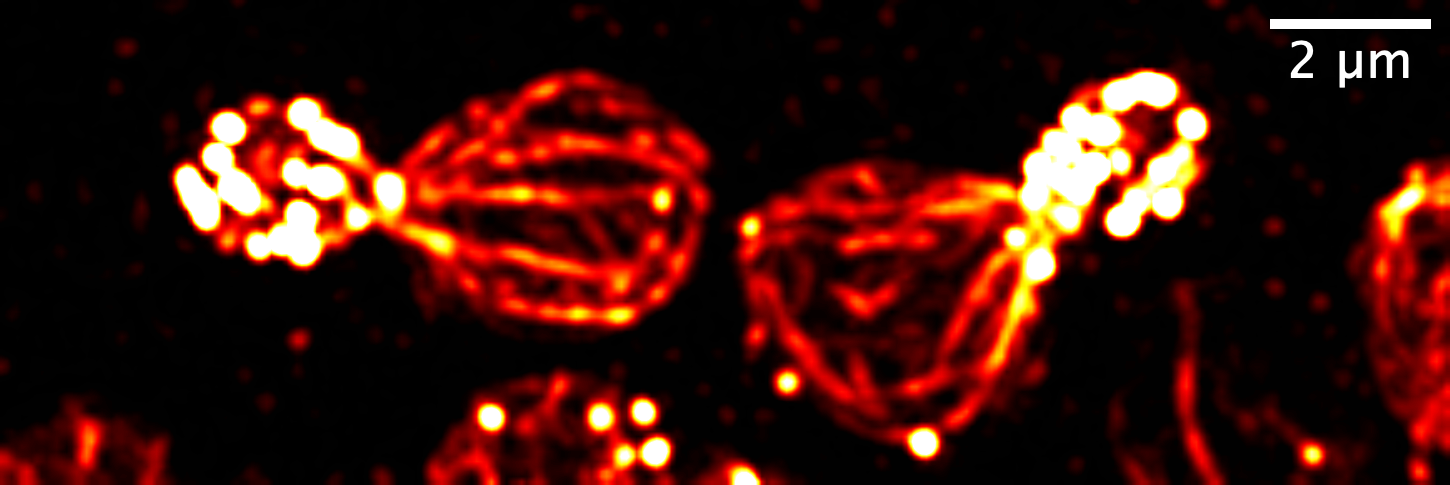
Fluorescent microscopy image of yeast cells showing actin filaments (“highways”) which acts as tracks for transport of supplies which are protected by the Tropomyosin type Tpm2, in the absence of the other Tropomyosin type, Tpm1 (Image: Anubhav Dhar, Bagyashree VT, Saravanan Palani)
REFERENCE:
Dhar A, Bagyashree VT, Biswas S, Kumari J, Sridhara A, Subodh J, Shekhar S, Palani S, Functional redundancy and formin-independent localization of tropomyosin isoforms in Saccharomyces cerevisiae, PLOS Genetics (2025)
https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1011859
LAB WEBSITE:
https://syncellbiolab.weebly.com/





