29 September 2025
– Rohini Subrahmanyam
Lithium-ion batteries power most electronics, but they have limited energy density – they can store only a certain amount of energy per mass or volume of the battery. “In order to store even more energy with the same mass or volume, you will have to explore alternative energy storage technologies,” says Sai Gautam Gopalakrishnan, Assistant Professor at the Department of Materials Engineering, IISc.
Gopalakrishnan and his team have studied how to boost the movement of ions in magnesium batteries, which can have a higher energy density. In a new study, using a machine learning model, they show that using amorphous materials as positive electrodes to build these batteries can significantly increase their rate of energy transfer.
Lithium ion or magnesium batteries contain a positive (cathode) and a negative (anode) electrode, separated by a liquid electrolyte. Each time a lithium or magnesium ion goes from the cathode to the anode or vice versa, energy is exchanged with the device. “In magnesium batteries, each magnesium atom can actually exchange two electrons, whereas each lithium atom can only exchange one electron with the external circuit. So, you can get close to twice the amount of energy per atom moved,” explains Gopalakrishnan.
The cathodes need to act like a sponge – upon applying an external potential, they should absorb and release magnesium ions into the electrolyte. But the main bottleneck in commercialising magnesium batteries is the lack of good materials that can act as cathodes, Gopalakrishnan says. So far, scientists have largely been looking at crystalline materials, which have a periodically ordered arrangement of atoms. However, because magnesium moves very slowly within these materials, they are unable to absorb and release magnesium ions at a fast enough rate.
”If we break the crystallinity and create something that is amorphous, haphazard, and chaotic, that may actually help magnesium to move fairly well within the structure,” Gopalakrishnan explains.
The team built a computational model of an amorphous vanadium pentoxide material and calculated how fast magnesium ions can move within it. To build such models, scientists typically use a method called density functional theory (DFT), which accurately models systems at an electronic level. But it takes a long time to simulate amorphous systems using this method. Molecular dynamics (MD) simulations – in which one studies interactions between atoms – are faster but less accurate. “Modelling amorphous systems accurately is very difficult,” says Vijay Choyal, first author of the study and a former postdoctoral scholar at IISc.
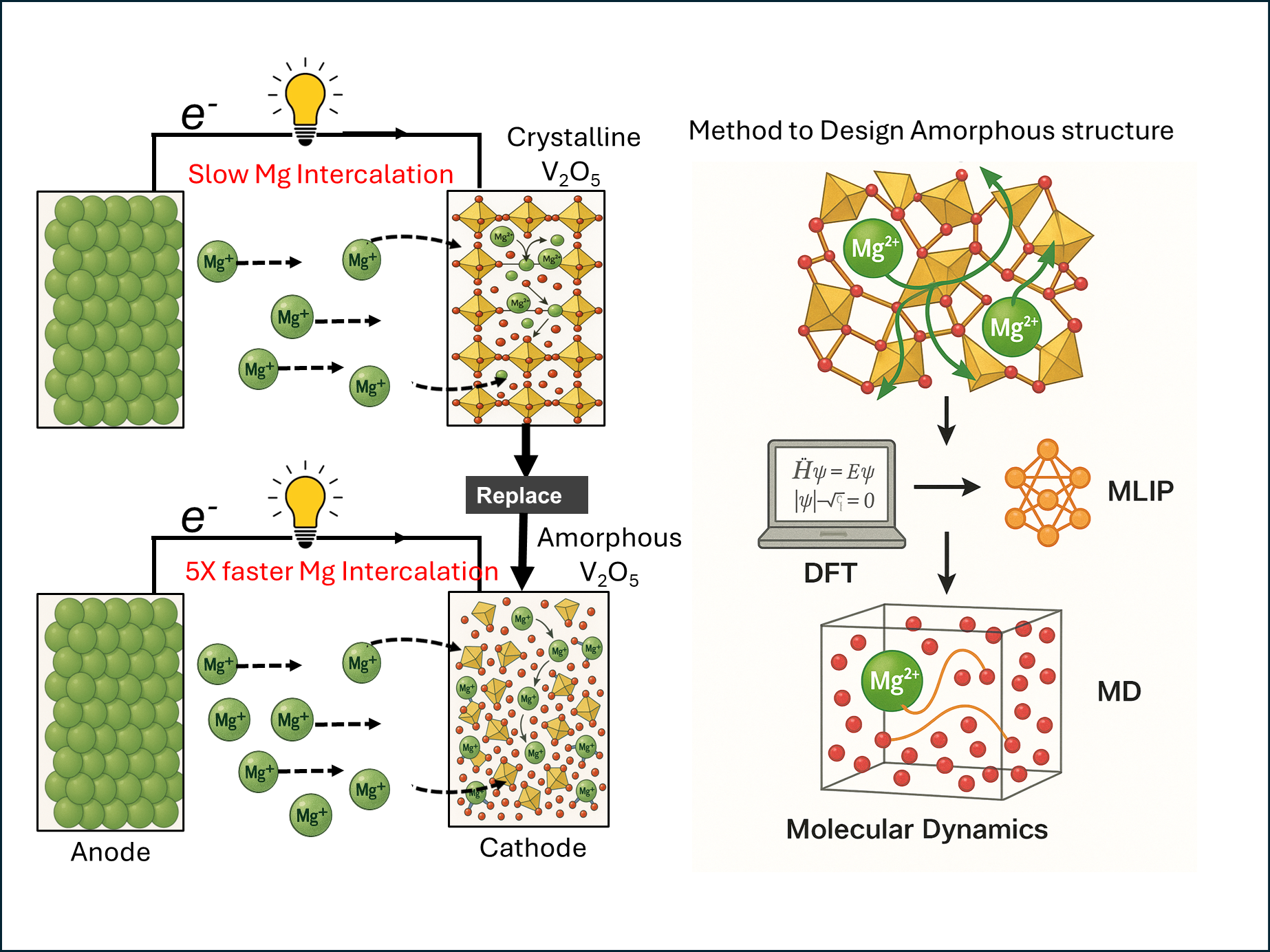
Making ions move faster by making structures amorphous (Image: Vijay Choyal)
To combine speed and accuracy, the team used a machine learning framework. They first used DFT to generate data on how the amorphous cathode would function at a small scale. After training their machine learning model on this data, they used the model to perform MD simulations. With MD, they were able to model the material at a larger scale – to get a better picture of how far the magnesium moves within the amorphous material and how long it takes. Compared to state-of-the-art crystalline magnesium materials, the team observed about five orders of magnitude improvement in the rate of magnesium movement in the amorphous form.
“Our work offers a completely different pathway to identify electrode materials for batteries and takes us a step closer to commercialisation of magnesium batteries,” says Gopalakrishnan.
The team hopes that experimentalists can now work on this amorphous material and test its effectiveness in the lab. “One disadvantage is that we don’t know how stable the amorphous materials can be when used in a practical battery,” says Debsundar Dey, co-author of the study and former MTech student at IISc. “The key takeaway is that using amorphous materials increases the mobility of ions, but we also need to experimentally validate our observations.”
REFERENCE:
Choyal V, Dey D, Gopalakrishnan SG, Exploration of Amorphous V2O5 as Cathode for Magnesium Batteries, Small (2025).
https://onlinelibrary.wiley.com/doi/10.1002/smll.202505851
CONTACT:
Sai Gautam Gopalakrishnan
Assistant Professor
Department of Materials Engineering
Indian Institute of Science (IISc)
Email: saigautamg@iisc.ac.in
Phone: +91 80 2293 2342
Website: https://sai-mat-group.github.io/
NOTE TO JOURNALISTS:
a) If any of the text in this release is reproduced verbatim, please credit the IISc press release.
b) For any queries about IISc press releases, please write to news@iisc.ac.in or pro@iisc.ac.in.


